Download the full application note by clicking here (PDF)
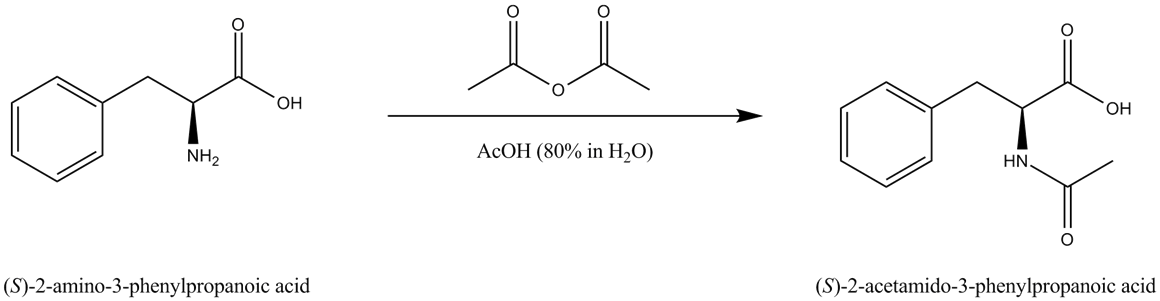
The reaction of L-Phenylalanine with acetic anhydride
Online monitoring of chemical reactions is a fast-growing field finding applications in R&D labs, pilot plants, and large chemical production plants where fully automated analyzers provide feedback to control the reactor. Today, mostly optical techniques like near-infrared and Raman spectroscopy are applied online due to their simple implementation using immersion probes directly mounted within reaction vessels or pipes. However, these methods require complex data analysis based on multivariate techniques like partial least squares regression (PLS-R), and demanding calibrations valid for all states during the reaction. NMR spectroscopy has the advantage of being an absolute method. The direct proportionality of the NMR signal amplitude to the concentration of the molecules in the sample allows for an absolute quantification without the need for calibration and is independent of the composition of the sample or the solvent used in the preparation. With the availability of the Spinsolve compact benchtop NMR systems, the NMR spectrometer can be brought directly next to the reaction setup in the lab or even in plant.
Permanent magnet systems do not need cryogens or special maintenance and only minimal training is required for personnel to perform the measurements due to the ease of use. In this application note we show the performance of a Spinsolve 60 MHz ULTRA to monitor the N-Acetylation of L-Phenylalanine with acetic anhydride (Fig. 1). L-Phenylalanine is an essential amino acid used in a variety of industrial processes, e.g. as reactant in the synthesis of the ACE-inhibitor Alacepril, as well as in the production of the well-known, non-saccharide sweetener, Aspartam. In many chemical processes involving amino acids the reactive centers must be protected to ensure the required regioselectivity of the reaction. In this example the acetylation of the amine works as a protection group to move the reactive center towards the acid functionality of the L Phenylalanine. Besides monitoring the progress of the reaction, the NMR spectra also provide information about the addition of reactants step and the consequent hydrolysis of the reactant acetic anhydride.
NMR spectra of reactants and products
The first step prior to monitoring a chemical reaction is to identify the regions in the NMR spectrum that can be integrated to quantify the concentration of reactants and products. Figure 2 shows the NMR spectra of L-Phenylalanine (bottom) and N-Acetyl-L-Phenylalanine (top), where one can quickly identify that the regions corresponding to the CH-group in the alpha position for the amine are good candidates. The region used to quantify L-Phenylalanine is marked in blue (3.8–4.2 ppm) and the one used for N-Acetyl-L-Phenyl-alanine is marked in red (4.2–4.8 ppm).
NMR spectra of reactant (bottom) and product (top) measured in a 5 mm NMR tube.
Download the full application note by clicking here (PDF)
Experimental setup
The reaction was carried out in a three-necked flask installed in a standard laboratory fume hood. L-Phenylalanine (1.65 g, 0.01 mol, 1 Eq.) was dissolved in 16.5 mL of acetic acid (80 wt-% in H2O) acting as the solvent. About 3 ml of acetic anhydride at room temperature was added continuously within 40 minutes by using a peristaltic pump. The mixture was stirred with a magnetic stirrer and monitored for an overall time of 2.5 h. To monitor the reaction online a Spinsolve 60 MHz Ultra system was placed directly next to the reactor. The reaction mixture was pumped from the reactor to the spectrometer and back to the reactor by using a second peristaltic pump. A glass flow cell going from the bottom to the top of the spectrometer, connected to the reactor in a closed-loop as shown in was used to keep the reaction mixture flowing through the NMR system with a constant flow rate of 0.8 mL/min.