In an earlier post on qNMR we described how benchtop NMR can be used to quantify the concentration of a sample or measure its purity. When such quantitative methods are validated, there are standard requirements for accuracy, precision, range, and linearity over that range that need to be met.
For example, the United States Pharmacopeia (USP) specifies the general requirements for a Category I NMR method when measuring a drug substance (there are other specifications for finished products and impurities). These specifications are listed in the table at the end of this post and are compared to the measured Spinsolve performance.
We have validated the Spinsolve benchtop qNMR performance by measuring the purity of one reference standard, methylsulfonylmethane (MSM), with another, maleic acid. Maleic acid is a common reference standard for qNMR, so this was used as the reference to measure the known purity of MSM (specified 99.5% pure). A spectrum of the mixture in D2O is shown in Figure 1.
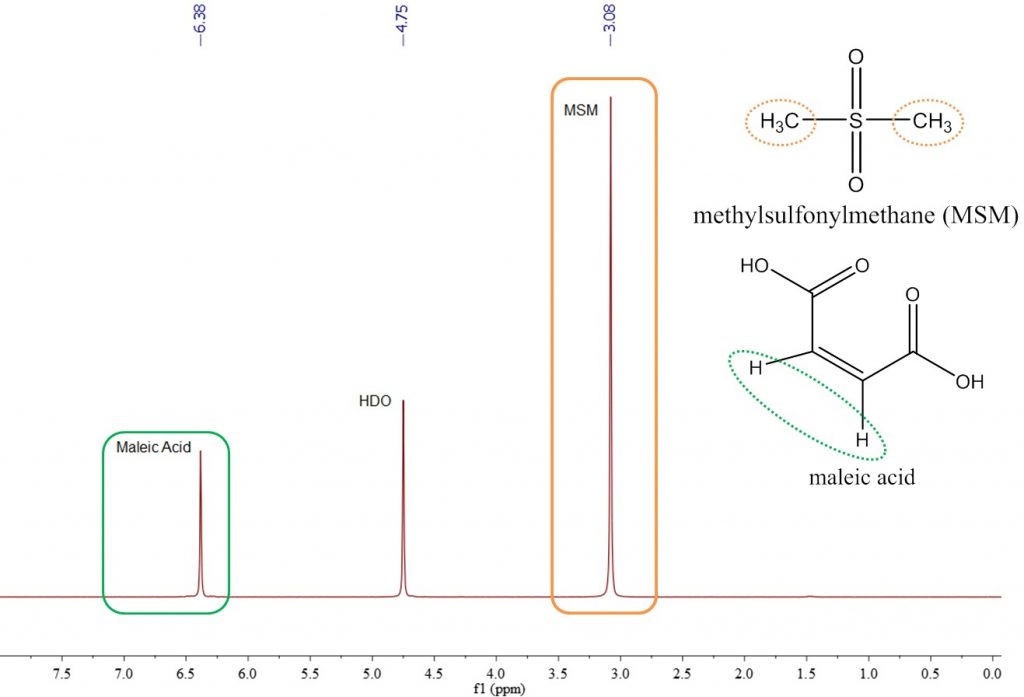
Maleic acid and MSM were co-dissolved in D2O at a concentration of approximately 60 mg/mL. The maleic acid peak is a singlet, has two protons, and has a chemical shift of about 6.3 ppm. The methyl peak of MSM is a singlet representing six protons and located at 3 ppm. The purity was determined from these peaks to assess the accuracy and precision of this method.
Let us recall the difference between accuracy and precision:
- Accuracy is the proximity of measurement results to the true value.
- Precision is the repeatability, or reproducibility of the measurement.
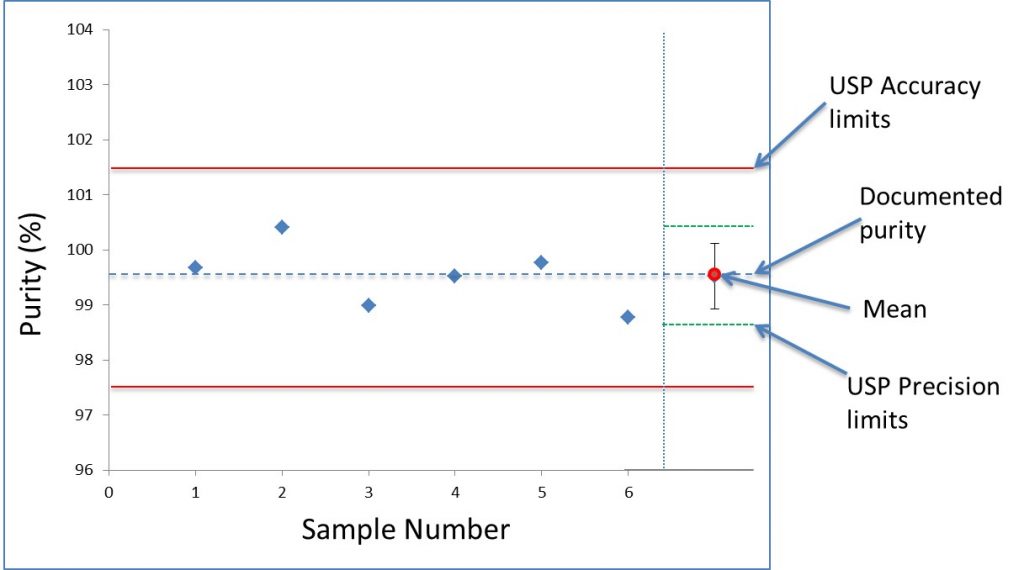
Figure 2 shows the purity measured by NMR for six independently prepared maleic acid and MSM solutions. The mean purity and standard deviation measured for MSM was 99.5 ±0.6%. This shows an accuracy within 2% and repeatability (precision) of less than 1%. The total uncertainty of these results is a combination of uncertainties in the measurement, as well as for the preparation of the samples. The error budget for weighing the samples is about 0.3%, or half of the total uncertainty.
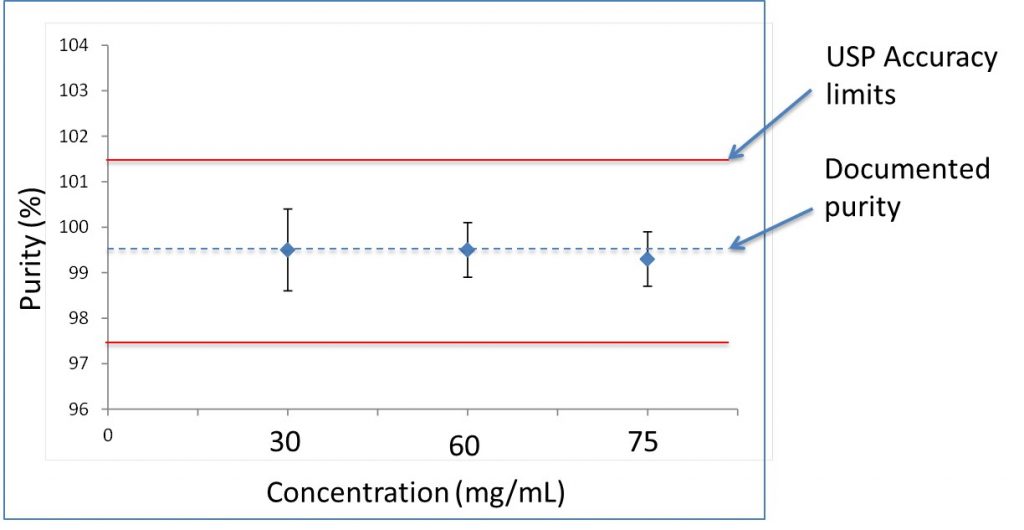
This purity measured for six independently prepared maleic acid and MSM solutions was repeated for concentrations of 30 and 75 mg/mL (50-120% of 60 mg/mL). Over this range the accuracy and precision were 0.9, 0.6, and 0.6%, which is shown in Figure 3.
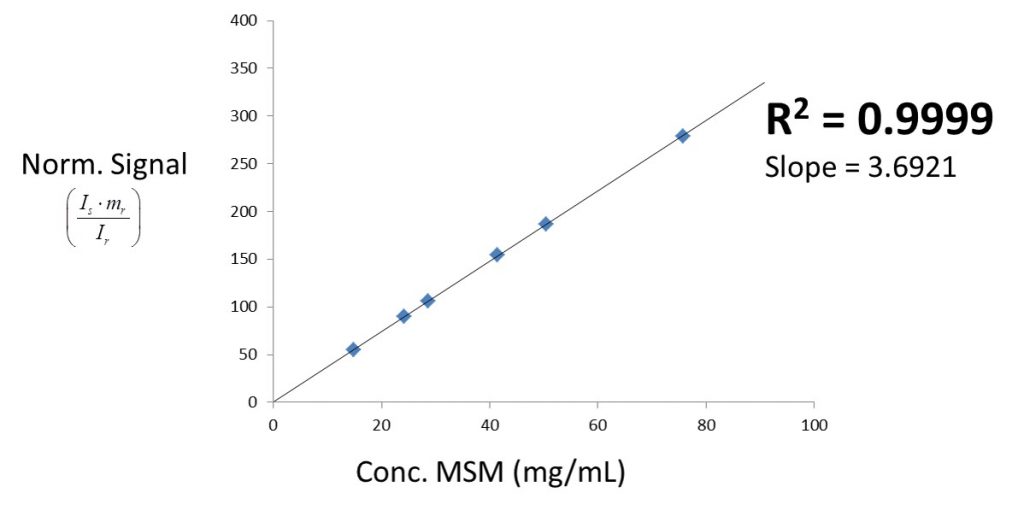
The linearity test between 15 mg/mL and 75 mg/mL is shown in Figure 4. A correlation coefficient (R2) of 0.9999 was determined, confirming highly linear behaviour between peak integral and concentration.
| USP Specification | Measured | |
| Accuracy | 98-102 % | 100 % |
| Repeatability | 1 % | 0.6 % |
| Range | 80-120 % | 50-125 % |
| Linearity | 0.995 | 0.999 |
USP <761> Nuclear Magnetic Resonance / Physical Tests
The results of these experiments are summarised in the table above. It shows that our benchtop qNMR method meets the accuracy, repeatability, range, and linearity requirements for a typical measurement of a drug substance.
Want to learn more about benchtop qNMR? Let us know!